Table of Contents
Introduction
Chemistry may seem complicated at first, but when broken down, it’s actually quite simple. You don’t need to be a scientist to understand how everyday elements react. In this article, we’re going to look at the chemical formula hcooch ch2 h2o and what it means. These letters and numbers represent a small chemical reaction, often seen in basic organic chemistry. Don’t worry if this looks confusing right now. By the time you finish reading, you’ll understand what it means, how the reaction works, and where it shows up in real life. We’ll walk through each component step by step. Whether you’re a student trying to study better or just someone curious about science, this article is for you. Learning chemistry doesn’t have to be challenging—it can actually be fun.
What Do These Letters Stand For?
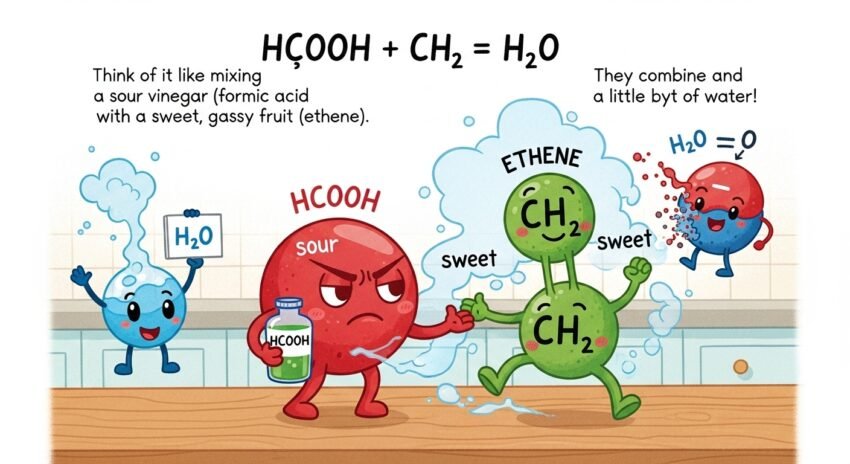
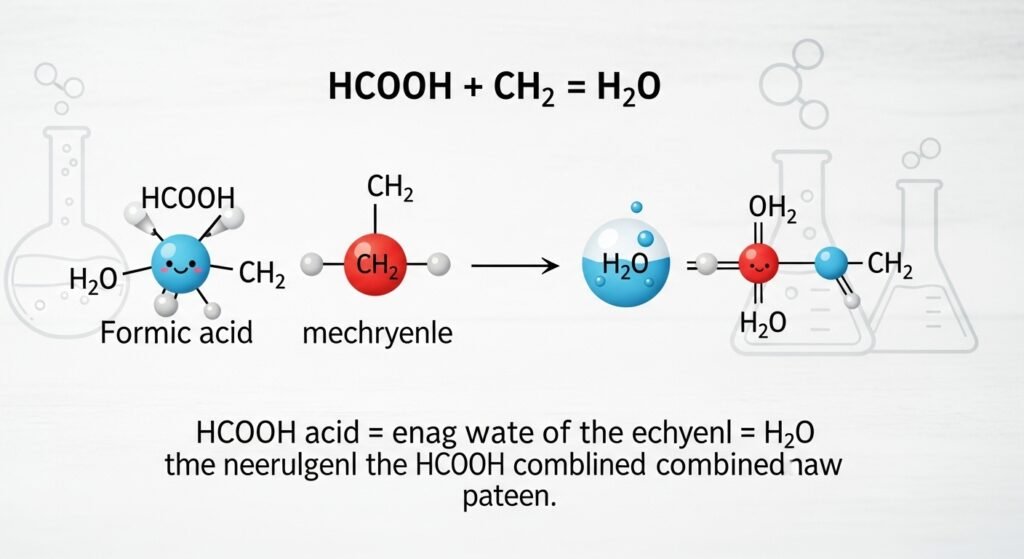
Let’s begin with the pieces in hcooch ch2 h2o. First, HCOOH is a form of formic acid, which is one of the simplest organic acids. It’s found in some insects like ants and also occurs naturally in the environment. CH₂ usually refers to a methylene group, a basic building block in many organic molecules. Lastly, H₂O is water—something we’re all very familiar with. So, this chemical formula is about how an acid, an organic group, and water can possibly react or interact in chemical steps. Don’t worry if it sounds hard. We’re going to dig into each part carefully so it all becomes clear.
What Is Formic Acid (HCOOH)?
Formic acid (HCOOH) is the simplest type of carboxylic acid. It has just one carbon atom. That’s why it has a short name and simple structure. This acid occurs naturally in some insects. Ants, for example, use it as a defense chemical. Some plants contain it as well. You can also find formic acid in some cleaning products and preservatives. It’s useful in industry but can also be studied in basic chemistry classes. In liquid form, it’s slightly smelly and can sting your skin. But in controlled labs, it plays a huge role in learning about how molecules interact.
Understanding the CH₂ Group
The CH₂ group, called a methylene group, consists of one carbon atom and two hydrogen atoms. It is often found within larger molecules. CH₂ groups help form the backbone of many organic compounds, including fuels and plastics. In this case, CH₂ could be part of a reaction combining small molecules into something bigger or splitting something apart. CH₂ can act like a bridge, helping one substance connect with another. In simple reactions, it may act as a helper or reactant depending on what else is in the mix. Understanding CH₂ helps make sense of how molecules stick together or break apart in reactions including hcooch ch2 h2o.
Why Water (H₂O) Is Important in Chemistry
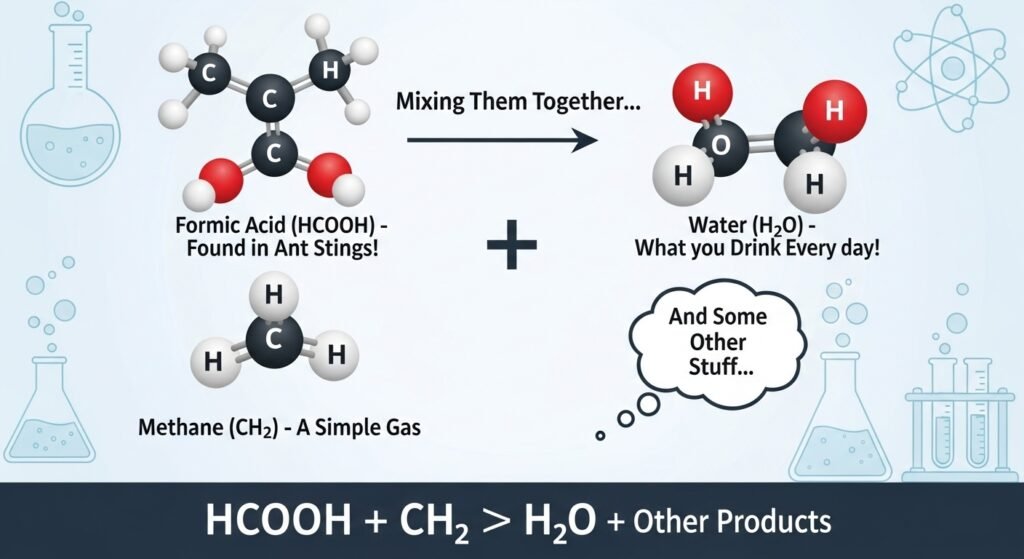
Water, also written as H₂O, is more than just something we drink. It’s a key part of many chemical reactions. In the case of hcooch ch2 h2o, water can act as a solvent, meaning it helps mix or stir the molecules. It also plays a role in reactions called hydrolysis, where water breaks apart larger molecules. Water may look simple, but it’s full of energy. Its structure lets it help acids, bases, and other groups react in special ways. In classrooms and labs, it’s rare to do chemistry without water being part of the setup.
Possible Reactions Between HCOOH and CH₂
So what happens when you mix HCOOH, CH₂, and H₂O? This depends on what form CH₂ comes in. If CH₂ is part of a compound like formaldehyde (HCHO), then the mix with formic acid and water might produce methanol (CH₃OH) in some conditions. In some reactions, especially in organic chemistry, formic acid acts like a reducing agent — helping other molecules lose oxygen or gain hydrogen. While this explanation is simplified, the main idea is this: these three parts can react under the right heat, pressure, and catalyst to form new, useful organic compounds. Some are used in plastic production. Others make cleaning materials. And some even help study how energy moves in natural life systems.
Real-World Uses of Formic Acid Reactions
Reactions like hcooch ch2 h2o happen in real labs all the time. These reactions are used to develop preservatives for food, make softening agents for leather, or aid in fuel cell energy systems. Yes, formic acid even has a place in green energy technology. In some new fuel cells, it is used to store hydrogen, making it easier and safer to use in cars and devices. Simple formulas like HCOOH mixing with other chemicals are behind some of the most exciting inventions in science. Knowing how these parts interact can lead to better, cleaner, more useful products in the future.
How These Chemicals Act Under Heat
When testing hcooch ch2 h2o in a lab, heat plays a big role. When formic acid is heated with certain chemicals, it breaks apart into gases like carbon dioxide and hydrogen. Adding a compound with CH₂ can change this process or create even more useful combinations. Heat increases the activity of the atoms, making it easier for bonds to form or break. That’s why even in beginner science classes, students learn to apply heat to reaction setups. Understanding thermal behavior in formic acid-based reactions helps predict what will happen and what products to expect.
The Balancing of the Equation
Though hcooch ch2 h2o looks like a reaction, it’s not a complete balanced equation. In actual chemistry, we would need more details—such as the specific CH₂ compound being used, whether a catalyst is present, or what temperature it’s under. For example, if CH₂ is part of methylene glycol (a hydrated form of formaldehyde), then things change completely. So when students or learners try to balance these equations, they would usually write a full chemical reaction and ensure the same number of atoms appear on both sides. This exercise teaches how chemistry honors mass and predictability. It’s not guessing—it’s numbers meeting nature.
How the Reaction Could Happen in Nature
In basic nature-based chemistry, hcooch ch2 h2o-like combinations can occur in microscopic systems. For instance, decaying plants release simple organic compounds, including acids like HCOOH. These can mix with bits of CH₂ compounds from soil microbes or old plant materials, along with rainwater or groundwater. Though it’s much slower than lab reactions, nature does its own chemistry. Over time, these natural mini-reactions affect soil nutrient levels and support bacteria or fungi growth. So what happens in a glass lab tube can also slowly happen under your feet in a forest.
Safety When Handling These Chemicals
Working with chemicals like hcooch ch2 h2o in a lab means you should be careful. Even though they sound simple, strong acids like formic acid can irritate your skin and eyes. Make sure you wear gloves, use goggles, and follow safety steps during any experiment or demo. In schools, teachers are trained to explain safety first, which is the right way to work. Even water, though harmless to drink, affects reactions when misused—it can splash, boil, or interact with other chemicals when heated. So whether you’re watching or working, respect every part of the experiment. Safety keeps science fun and risk-free.
Learning Chemistry Through Simple Reactions
The formula hcooch ch2 h2o may look small, but learning about it opens up big ideas. You start asking questions: What happens if we change this part? Does ice or steam affect the mix? Could these react better in air or sealed jars? This curiosity is the first step toward becoming a great student of chemistry. Once you learn the basics with a simple mix like this, bigger ideas become easier too—like acids, bases, esters, and carbon chains. So don’t let chemical names scare you away. Behind every formula is a puzzle waiting to be solved.
Conclusion
Understanding hcooch ch2 h2o isn’t just about memorizing letters and numbers. It’s about seeing how chemistry works in daily life and labs. From formic acid’s role in nature to how water and CH₂ compounds interact, this small formula helps open the door to deeper thinking. Whether you’re learning the parts, the reaction types, or just exploring how molecules move, this example gives you a solid base. It’s great for students, curious minds, and even science hobbyists. So next time you see a formula like hcooch ch2 h2o, don’t scroll away. Dive into it. You’ll be surprised just how much science fits into a few short symbols.
FAQs
What does hcooch ch2 h2o mean in chemistry?
It refers to a group of compounds: formic acid (HCOOH), a CH₂ group (often part of another compound), and water.
Is this a full chemical equation?
Not fully. It shows reactants, but the products depend on precise conditions and experimental setup.
Where is formic acid used in real life?
Formic acid is used in cleaning, agriculture, leather processing, and even modern energy fuel cells.
Can I mix these chemicals safely at home?
No, formic acid is a strong acid and needs proper lab handling with safety equipment and supervision.
Is water always involved in chemical reactions?
No, but it’s involved in many reactions as a solvent, reactant, or product, especially in organic chemistry.
Why is this reaction important to study?
Studying reactions like hcooch ch2 h2o helps learners understand acids, organic reactions, and key chemistry skills.